CANDU reactors
By Dr. Nick Touran, Ph.D., P.E., , Reading time: 1 minute
 CANada Deuterium Uranium (CANDU) reactors use heavy water as a coolant and
moderator instead of regular old light water. These
were invented and developed in Canada in the 1960’s, when Canada decided that
they did not want to build enrichment plants or large pressure vessel
manufacturing capabilities. Heavy water can be made from regular water, but it
takes a lot of energy to do so. But, heavy water absorbs neutrons much less
probably than regular water, so it’s significantly easier to sustain a
neutron chain reaction with heavy water. This allows CANDU reactors to operate
with natural uranium (no enrichment necessary). As of 2010, there are 29 operating
CANDU reactors, with 17 of these in Canada.
CANada Deuterium Uranium (CANDU) reactors use heavy water as a coolant and
moderator instead of regular old light water. These
were invented and developed in Canada in the 1960’s, when Canada decided that
they did not want to build enrichment plants or large pressure vessel
manufacturing capabilities. Heavy water can be made from regular water, but it
takes a lot of energy to do so. But, heavy water absorbs neutrons much less
probably than regular water, so it’s significantly easier to sustain a
neutron chain reaction with heavy water. This allows CANDU reactors to operate
with natural uranium (no enrichment necessary). As of 2010, there are 29 operating
CANDU reactors, with 17 of these in Canada.
Pros
- No enrichment required. Heavy water is such an excellent moderator that it allows natural uranium to be burned directly
- Bypassing enrichment, no depleted uranium tails are made. This allows CANDUs to get very efficient use of the uranium resource, higher than all other water-cooled reactors
- CANDUs can be refuelled without shutting down the reactor, avoiding the downtime that most other reactors require
Cons
- Heavy water is expensive
- When regular water absorbs one neutron, it becomes heavy water. But when heavy water absorbs one neutron, it becomes tritium (H-3), which is a low-level radioactive hazard. Tritium is difficult to contain and enters biological systems readily. CANDUs make more tritium than light-water reactors because they have so much heavy water.
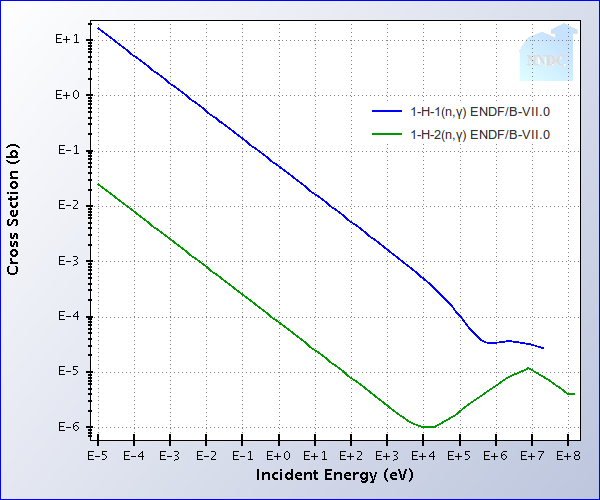 In this figure, we plot the probability that hydrogen and deuterium will absorb a neutron (hurting
the chain reaction). As you can see, H-2 (deuterium, the constituent of heavy water) eats up
neutrons with 3 orders of magnitude less probability.
In this figure, we plot the probability that hydrogen and deuterium will absorb a neutron (hurting
the chain reaction). As you can see, H-2 (deuterium, the constituent of heavy water) eats up
neutrons with 3 orders of magnitude less probability.